
The Underlying Changes in Serum Metabolic Profiles and Efficacy Prediction in Patients with Extensive Ulcerative Colitis Undergoing Fecal Microbiota Transplantation
The crucial role of gut microbiota in ulcerative colitis (UC) makes microbial manipulation an attractive treatment for UC. Increasing evidence from randomized controlled trials has suggested that fecal microbiota transplantation (FMT) is an effective and safe treatment for active UC. However, the underlying mechanisms and mediators of FMT remain unclear. Moreover, the heterogeneous clinical outcomes of FMT for UC necessitate a personalized treatment strategy to optimize efficacy and reduce costs. Metabolomic techniques provide insights into disease pathogenesis and therapeutic response assessment by elucidating disease-associated metabolic perturbations and changes in host metabolism due to therapeutic intervention, which is helpful in clinical decisions making. Recently, the journal Nutrients published a clinical study by our team on ” The Underlying Changes in Serum Metabolic Profiles and Efficacy Prediction in Patients with Extensive UC Undergoing FMT “. Here, untargeted metabolomic analysis by liquid chromatography–mass spectrometry (LC–MS) was used to broadly assess metabolic changes and identify potential biomarkers in patients with UC treated by FMT. Our analysis will provide a reference for quantitative measurement of the identified biomarkers and then timely monitoring of the efficacy of FMT in the future, thereby laying the foundation for the establishment of a high- performance artificial intelligence evaluation model.
This study included 55 UC patients, 11 of whom were excluded for reasons described in Figure 1. Therefore, 44 patients with active UC were included in the analyses. Clinical efficacy was evaluated by partial Mayo Scores 3 months after FMT. Clinical response was defined as a partial Mayo Score decrease ≥3 and ≥30% from baseline, in addition to a decrease ≥1 in the rectal bleeding sub-score or a final rectal bleeding sub-score ≤1. Clinical remission was defined as a partial Mayo Score ≤1. Serum samples of participants before and 1 week after FMT were collected for LC-MS analysis. The main findings were shown as following:
(1) FMT successfully induced the clinical response (50.0%, 22/44) and clinical remission (29.5%, 13/44) three-month post-FMT in the selected population with extensive UC refractory to conventional treatments.
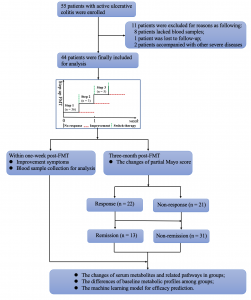
(2) In the response group, the top two significantly altered pathways (Figure 2, p < 0.05) were vitamin B6 metabolism (4-pyridoxic acid, pyridoxal, 4-(phosphonooxy)-threonine), and aminoacyl-tRNA biosynthesis (L-lysine, L-serine, L-histidine, L-glutamine, and L-methionine). The serum metabolome profiles in the remission and response groups were partially improved after FMT when compared with the non-remission and non-response groups.
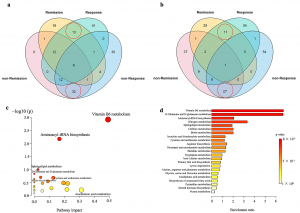
(3) A clear separation was found between the remission and non-remission groups at baseline in the positive ion mode of serum metabolome profiles.
Using the Boruta method, we selected 20 metabolites that was optimally relevant features useful for remission prediction from a total of 2148 as variables for random forest (RF) modeling. The AUC of the RF classifier was 0.963 (Figure 3), supporting the potential role of serum metabolites in predicting clinical remission following FMT in UC patients.
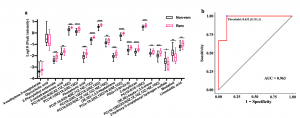
(4) In the remission group, glycerophosphocholines (GPCs), glycerophospholipids, and glycerophosphoethanolamines were higher than those in the non-remission groups. The prominent role of GPCs in the predictive analysis provides further support that they improve FMT efficacy. Therefore, metabolome-informed FMT, for example, via oral phosphatidylcholine supplementation before FMT, may provide enhanced therapeutic effects.
These findings suggest that the efficacy of FMT for patients with extensive refractory UC may be related with the improvement of host metabolomics. A promising role of serum metabolites in the non-invasive prediction of FMT efficacy for UC demonstrated the value of metabolome-informed FMT in managing UC. However, this is a retrospective analysis of a prospective trial with a small sample size, and the findings are necessary to be validated in a large-scale and well-designed investigation in the future.
Reference:
Wu, X.; Li, P.; Wang, W.; Xu, J.; Ai, R.; Wen, Q.; Cui, B.; Zhang, F. The Underlying Changes in Serum Metabolic Profiles and Efficacy Prediction in Patients with Extensive Ulcerative Colitis Undergoing Fecal Microbiota Transplantation.Nutrients 2023,15, 3340(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10421017/)


